Section: New Results
CREST: Chemical Reactivity Exploration with Stochastic Trees
Participants : Leonard Jaillet, Stephane Redon.
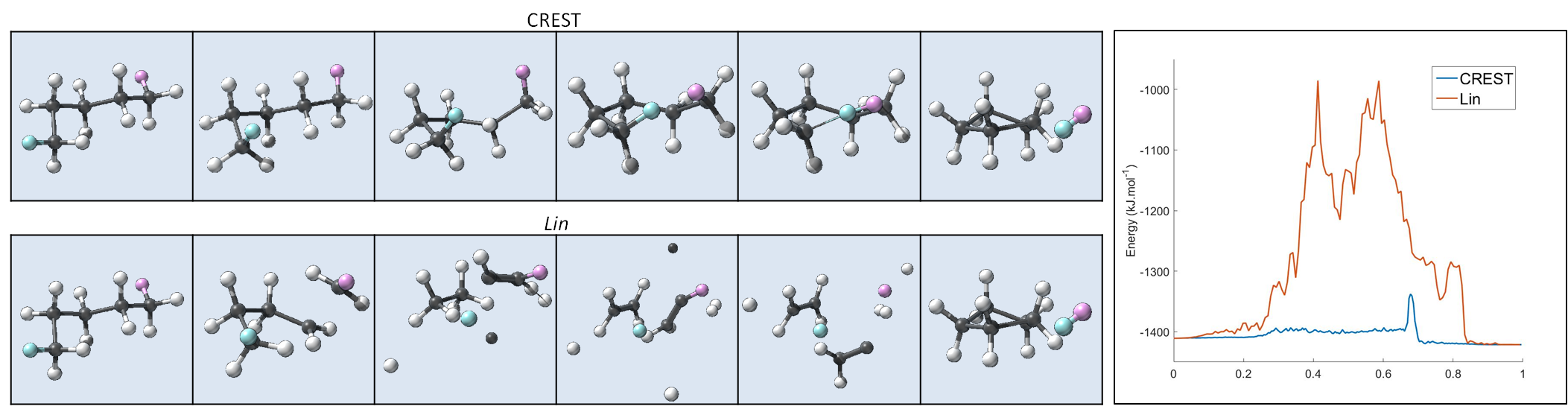
We have proposed the CREST method (Chemical Reactivity Exploration with Stochastic Trees), a new simulation tool to assess the chemical reaction paths of molecular systems. First, it builds stochastic trees based on motion planning principles to search for relevant pathways inside a system's state space. This generates low energy paths transforming a reactant to a given product. Then, a nudged elastic band optimization step locally improves the quality of the initial solutions. The consistency of our approach has been evaluated through tests in various scenarios. It shows that CREST allows to appropriately describe conformational changes as well as covalent bond breaking and formations present in chemical reactions (see figure 14).
This contribution appears in continuity of our previous work regarding the development of a geenric Motion planning architecture for nanosystems. Important features have been added to specifically treat the case of chemical reaction, such as structure alignment, exploration based on multiple trees, automatic resizing of the sampling volume, etc.
|